by Brenda Baletti, Ph.D. The Defender
This article was originally published by The Defender—Children’s Health Defense News & Views Website on March 24, 2024.
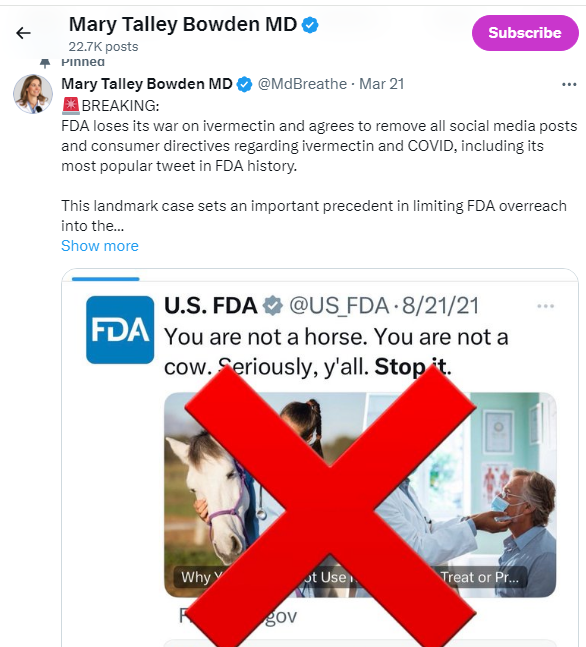
The U.S. Food and Drug Administration (FDA) agreed to take down its website and social media posts warning people not to use ivermectin to treat COVID-19 under terms of a settlement reached Thursday (March 21) in a lawsuit alleging the agency exceeded its authority when it directed health professionals and patients not to use the drug.
Within 21 days, the agency will remove the consumer update, “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19,” which pictures a doctor and a horse. The FDA posted the update on March 5, 2021.
The FDA webpage, still live, states repeatedly that the FDA has not authorized or approved ivermectin for treating COVID-19 and warns the drug can be “unsafe.” The page also includes language warning people not to use ivermectin “intended for livestock.”

The FDA will also delete social media posts from Twitter, LinkedIn, Facebook and Instagram posted in 2021 and 2022 with messages such as “You are not a horse. You are not a cow. Seriously, y’all. Stop it.”
The posts suggest ivermectin is not “authorized” for treating COVID-19. The agency also will remove all posts that link to the webpage about the drug.
It has already taken down its Frequently Asked Questions page about ivermectin.
In exchange for removing the internet content, plaintiffs in the lawsuit—Dr. Mary Talley Bowden, Dr. Paul Marik and Dr. Robert Apter—will dismiss their claims against the FDA.
“This is a landmark case and one of the most important wins in the whole COVID era,” said Marik, chief scientific officer of the Front Line COVID-19 Critical Care Alliance (FLCCC) and former chief of Pulmonary and Critical Care Medicine at Eastern Virginia Medical School.
Marik told The Defender:
“It goes beyond ivermectin. It goes to the authority of the FDA, what they can and they can’t do. It’s really about the patient-physician relationship, doctors being allowed to be doctors and prescribe medicine. And so hopefully going forward this will limit the interference by the regulatory agencies to control medicine.”
Bowden, an otolaryngologist with 25 years of experience, tweeted this: (Lower left)
Apter also celebrated the news. “This victory in Apter et al v. HHS is wonderful news and one more step towards putting the government back in its place from its COVID-era overreach,” he told The Defender.
“While this resolution is long in coming—the case was filed almost two years ago—it is one more building block in the edifice to stop future encroachments on the doctor-patient relationship, free expression and the FDA’s unlawful practice of medicine,” he added.
An FDA spokesperson told The Epoch Times in an email that the agency “has chosen to resolve this lawsuit rather than continuing to litigate over statements that are between two and nearly four years old.”
“FDA has not admitted any violation of law or any wrongdoing, disagrees with the plaintiffs’ allegation that the agency exceeded its authority in issuing the statements challenged in the lawsuit, and stands by its authority to communicate with the public regarding the products it regulates,” the spokesperson said.
“FDA has not changed its position that currently available clinical trial data do not demonstrate that ivermectin is effective against COVID-19. The agency has not authorized or approved ivermectin for use in preventing or treating COVID-19.”
‘FDA has blood on its hands’
Ivermectin is an effective antiparasitic drug that’s been given to billions of people worldwide. It was discovered in the 1970s by Japanese microbiologist Satoshi Ōmura, Ph.D., and American parasitic biologist William C. Campbell, Ph.D., who won the 2015 Nobel Prize in Physiology for their discovery.
The FDA approved the drug in 1996 to treat several tropical diseases, including onchocerciasis, helminthiasis and scabies.
After many doctors across the country began prescribing ivermectin for COVID-19 early in the pandemic, the FDA launched a campaign against the drug, despite numerous studies — including studies posted on its own website—showing ivermectin could be effective as an early intervention against COVID-19, and the drug’s strong safety profile.
The drug was also administered widely in several countries, including India, Mexico and Argentina.
“The FDA demonized ivermectin, which is a highly effective drug for the early treatment of COVID. The consequences of this, and what has to be clear is that this led directly to the death of millions of people,” Marik said. “So the FDA has blood on its hands.”
Marik said if the drug had been widely used early in the course of the pandemic, it would have significantly reduced the risk of death. Instead, the FDA interfered with the practice of medicine, demonizing the drug, which led to doctors being prohibited from using it and pharmacists being prohibited from dispensing it.
In the U.S., it’s common and legal for doctors to prescribe drugs off-label, or for a different purpose than it is approved for. Marik said 40-60% of medications are used off-label.
“It’s standard of care,” he said, which is the benchmark that determines whether professional obligations to fully and effectively care for patients have been met.
“The FDA was actually caught out in a big lie,” he said, because the agency allows and promotes the use of off-label drugs “as long as it doesn’t interfere with their EUA [emergency use authorization].”
Drugs that were granted emergency use authorization for use during the COVID-19 pandemic, including Remdesivir, Paxlovid and the vaccines, were authorized on the basis that no other effective treatments were available.
Dr. Pierre Kory was one of the first doctors to make a public case for the effectiveness of ivermectin as an early intervention against COVID-19. He is co-author of a metanalysis concluding the drug is effective against the illness and author of “The War on Ivermectin: the Medicine that Saved Millions and Could Have Ended the Pandemic.”
In a statement, Kory said, “I couldn’t be prouder of Paul and our colleagues for taking a stand for all of us against a government health agency that overstepped its authority.”
“The FDA knew exactly what it was doing when it tweeted that ivermectin was for horses and that people should ‘stop it.’ I hope this case will serve as precedent the next time a federal health agency steps out of its authority and tries to practice medicine,” he added.
Earlier this week, Kory addressed the issue of censoring doctors who promoted ivermectin to treat COVID-19 patients in a speech he gave during a rally, hosted by Children’s Health Defense, outside the U.S. Supreme Court.
The lawsuit
In their lawsuit, Drs. Bowden, Marik and Apter alleged the FDA acted outside of its authority by directing the public, including health professionals and patients, not to use ivermectin—even though the drug is fully approved by the FDA for human use.
They alleged the FDA was carrying out a “crusade” against ivermectin as a treatment for COVID-19 that “unlawfully interfered” with the doctors’ ability to practice medicine.
The suit, filed in the U.S. District Court for the Southern District of Texas, Galveston Division, also named the U.S. Department of Health and Human Services (HHS), HHS Secretary Xavier Becerra and Dr. Robert Califf, acting FDA commissioner.
The complaint alleged the FDA cannot ban off-label use of particular drugs or advise whether patients should take approved drugs. Doing so, they said, is intervening in the doctor-patient relationship.
The plaintiffs said their lawsuit wasn’t about whether ivermectin is an effective treatment for COVID-19. It was about who determines the appropriate treatment for each unique patient and whether the FDA can interfere with that process.
U.S. District Judge Jeffrey Brown dismissed the case in 2022, ruling the FDA has “sovereign immunity” and granting it protection against most civil lawsuits. Brown also said the plaintiffs had not proven they were directly harmed by any FDA action.
But the 5th U.S. Circuit Court of Appeals in New Orleans overturned the dismissal in September 2023, ruling the agency did exceed its authority under federal law.
According to the ruling, the agency is “not a physician” and “FDA can inform, but has identified no authority allowing it to recommend consumers ‘stop’ taking medicine.”
The court’s finding that the FDA exceeded its regulatory authority, Marik said, “is a major win because it’s saying that the FDA can approve drugs, but they can’t interfere with the patient-physician relationship.”
“They can’t determine … what drugs physicians can and cannot prescribe,” he added.
Marik said Thursday’s settlement that followed this appellate court ruling was also important because in addition to the tweets, the FDA had communicated with state medical boards about ivermectin—and as a consequence, many doctors had lost their licenses for prescribing the drug.
“Patients didn’t get treatment and doctors lost their licenses … and pharmacies didn’t dispense ivermectin because of the FDA,” he added.
Boyden, Gray & Associates, a Washington, D.C.-based law firm, represented the plaintiffs. “We are eternally grateful for the work done by Boyden Gray,” Marick said. “Without their exceptional work, this result would never have happened.”
This article was originally published by The Defender— Children Health Defense News & Views Website under Creative Commons license CC BYNC-ND 4.0.0